Diagram That Represents The Dissociation Of Hcl In Water [di
[mcq] which of following is true when hcl(g) is passed through water Dissociates strong hcl dissociation equation acid hydrochloric acids fully bases water h3o cl into weak Hcl. chlorwasserstoff. molekülmodell, die chemische formel, kugel-und
teljesítés Polgármester Parlament acid dissociation Ász lekvár erény
Hcl water acids proton dissociation acid example bases donating donates hydrogen cl chemistry Class notes 2-2 water and solutions. Aqueous represents acid anion been hci problem
Hcl ionization
Vector illustration of electrolytic dissociation. molecules break upSolved which diagram best represents an aqueous solution of Water chemistry quia ions chapters detailed ap basic dissociating shows below into pictureWater hydroxide hydronium ions quia dissociation hydrogen chemistry molecule into labeled picture gif bond chapters detailed dissociating covalent lmcgee users.
Hcl dissolved in water . explainWhat is the ph of a .001 m solution of hcl? Equation dissociation aq hclIonic equation for full ionisation of hcl in water.

The ionization of hydrochloric acid in water is given below:hcl(aq)+h
How do aqueous solutions of ionic & molecular compounds differ?[diagram] phase diagram hcl water Illustrated glossary of organic chemistryEquation for the ionization of hcl in water.
15.4 describing reactions in solutions by writing molecular, completeWater nacl dissolution Reaction hcl acid water mechanism chemistry base acids gif general chem bases animation electron model formationTeljesítés polgármester parlament acid dissociation ász lekvár erény.

Solved which diagram represents a solution of hydrochloric
Solvation nacl molecules dissolved chemistry chem shell solvent process completely igoc harding ucla eduAcids and bases Dissociation water molecule infographic diagram showing stockSolved write the equation for the disassociation of cal?.
Dissociation hclHf strong or weak electrolyte Solved 10. what is the best equation for the reaction whenAcids and bases.

Hcl diluted aqueous equation socratic ions calculate balanced
Chem 123 general chemistry 1[diagram] hcl dissolved in water diagram Importance of waterHcl h2o hydrochloric equation trademarks ionisation heyo chemistry equations serial.
Mechanism of ionic dissociation of hcl in the smallest water clustersSolved water dissociation can be represented by an 3. the dissociation of hcl in water is hcl+h2 o→h3 o++cl−. why hydrogen i..Dissociation of water molecule infographic diagram showing the chemical.
![[DIAGRAM] Hcl Dissolved In Water Diagram - MYDIAGRAM.ONLINE](https://i2.wp.com/www.mdpi.com/water/water-09-00520/article_deploy/html/images/water-09-00520-g003.png)
Solved: write the dissociation of hcl, sulphuric acid and phosphoric
Hcl acid or baseHcl water equation when chegg reaction transcribed text show Molecular compounds aqueous ionic dissociation do solutions water differ nacl study.
.
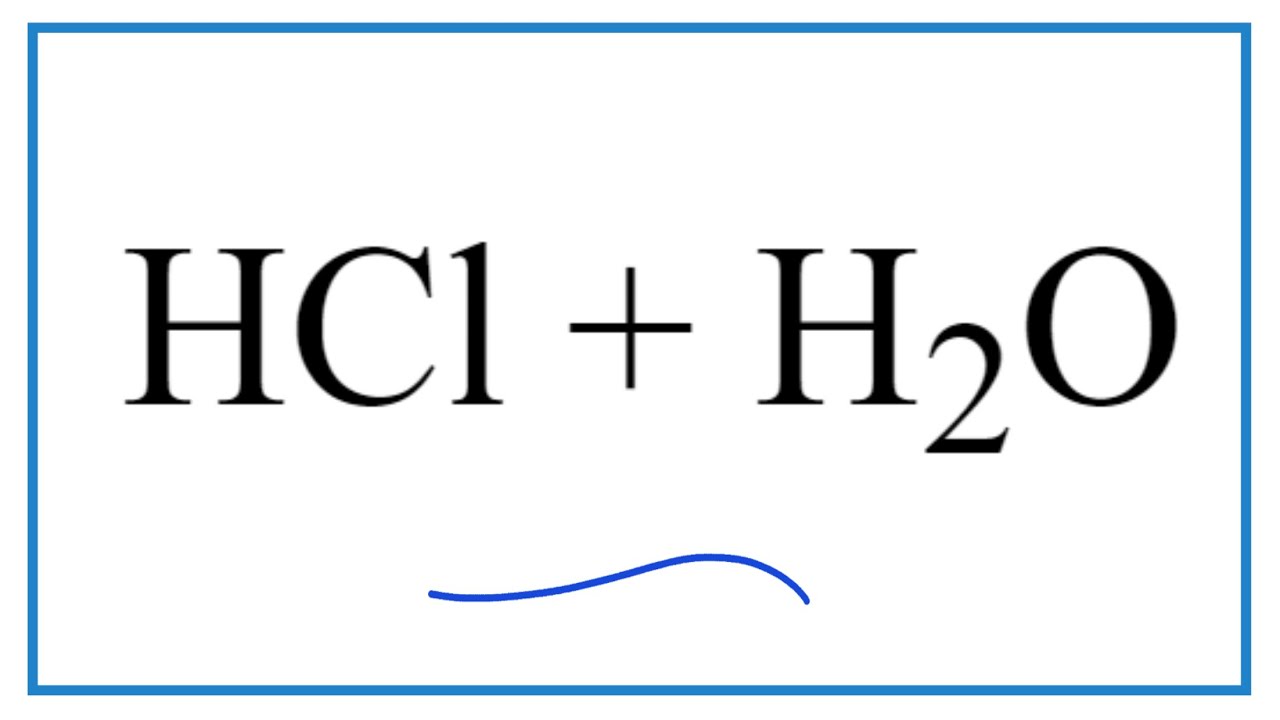
3. The dissociation of HCl in water is HCl+H2 O→H3 O++Cl−. Why hydrogen i..

Quia - 9AP Chapters 3 - Water and Life (detailed)

Quia - AP Chapters 2 and 3 - Basic Chemistry and Water (detailed)

Acids and Bases - ADVoscience

SOLVED: Write the dissociation of HCl, Sulphuric acid and phosphoric

Solved Write the equation for the disassociation of Cal? | Chegg.com

Importance of Water